Fisher & Paykel Launches Evora Full to the U.S.
The compact full-face sleep mask features the company’s Dynamic Support Technology and will be available May 9.
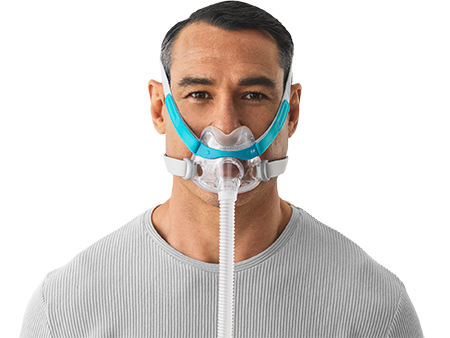
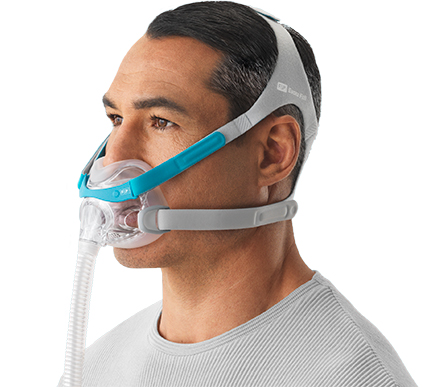
Sleep therapy equipment maker Fisher & Paykel Healthcare is launching its F&P Evora Full, a compact full-face mask for obstructive sleep apnea treatment, to the U.S. market.
Available for purchase by DME providers starting May 9, the Evora Full has received FDA 510(k) clearance.
“We welcome this clearance from the FDA and look forward to offering this unique full-face mask to our customers and patients,” said Justin Callahan, president of North America Operations for Fisher & Paykel Healthcare. “The Evora Full marks our entry into the compact full-face mask segment, and our teams have worked hard on achieving a final product that optimizes for both comfort and performance.”
The Evora Full features a floating seal and stability wings that work together to create what Fisher & Paykel has dubbed its Dynamic Support Technology. The compact seal floats under the nose to provide patients with a clear line of sight, while the stability wings keep the seal stable.
“Evora Full is a minimal-contact mask designed to allow patients to move and sleep freely without compromising performance,” said Andrew Somervell, vice president of Products and Technology for Fisher & Paykel Healthcare. “It offers patients full-face performance in a compact size.”
Clinical trial results for the Evora Full showed that:
- 91 percent of trial participants rated Evora Full as “stable” or “very stable.”
- 93 percent found the mask is “comfortable” or “very comfortable.”
- 96 percent could sleep in their preferred sleeping position.
The mask comes with three seal sizes (extra small, small to medium, and large) and two headgear sizes (standard and extra-large).
Fisher & Paykel Healthcare’s Evora Full video intro:
Images: